
Bismuth crystals have an otherworldly beauty, shimmering with iridescent colours that seem to dance across their stair-step surfaces when light hits them. Mistaking them for some kind of high-tech creation is easy, but they’re actually 100% natural. Their unique geometry – a real maze of spiraling edges and colors – has people wondering: what is it about these crystals, made from molten metal as they are, that gives them their otherworldly looks?
Let’s start with bismuth, a heavy (yet unusual) metal with a low melting point of around 271°C – low enough to melt on a cooktop if left to. Bismuth’s accessibility is one of the reasons it’s popular among backyard experimenters and scientists alike. And what happens when bismuth solidifies? It doesn’t just turn into a lump, as you might think. No, it forms “hopper” crystals, which are stepped formations that look like miniature ziggurats. So what’s going on here?
- INSPIRING SCIENCE FOR KIDS - You've come to the right place for a kids science kit with massive variety! Your kids will make bubbling, color-changing...
- A TOTAL OF 45 SCIENCE EXPERIMENTS FOR KIDS - The chemistry set itself comes with more than 15 experiments, but there's also a bonus guide with 30...
- EASY-TO-FOLLOW INSTRUCTIONS - Other science kits for kids wish they had an instruction booklet this fun! Every experiment has illustrated,...
It all comes down to how the atoms in bismuth arrange themselves as it cools from liquid to solid. It turns out the goal is to get to the most stable, lowest energy state possible. Think of free energy as internal stress in a material; atoms just want to relax in a state that requires the least amount of effort to hold together. And that’s exactly what happens to bismuth as it cools from a melt: the atoms shape the crystals in a certain way.
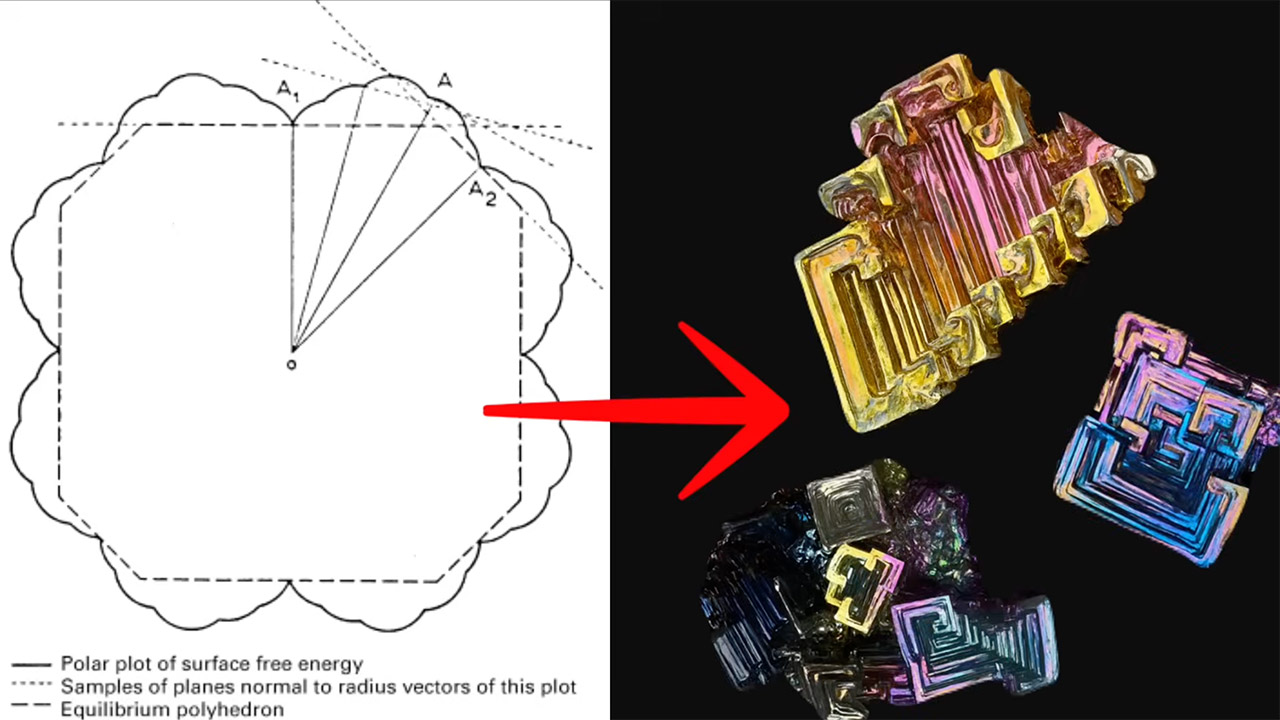
Bismuth crystals don’t form in a neat cube or prism shape from a supersaturated solution like salt or sugar crystals do; their conditions are far from equilibrium. So the atoms don’t have time to form a perfect, predictable shape. Instead, they prefer speed over stability, and that’s what we see.

When bismuth first starts to solidify, the atoms on the surface of the growing crystal have fewer neighbors to bond with than those in the bulk. This creates an energy cost near the edges, often called surface energy. To minimize this cost from getting too high, bismuth atoms will attach themselves to places with higher surface energy available, like at the corners and edges. These are the places where the crystal is less stable, and more welcoming to new arrivals. As a result the crystal grows faster in these areas than on the flat surface which is ideal for carving out the characteristic stepping, hopper-like structures that bismuth crystals are known for.

This is all about kinetics – the rate at which atoms solidify. In bismuth, the process happens fast enough that the atoms can’t build the neat rhombohedral shape. The perfect shape, a slightly distorted cube, only occurs when bismuth cools slowly and the atoms settle in. But in real life, where cooling takes minutes, the atoms rush to where they can bond the most – the high energy edges and corners. Each new layer of atoms repeats the process, stacking and spiraling outwards, giving the hopper crystal its complicated, terraced appearance.
Bismuth isn’t the only element that can make hopper crystals. Other materials from table salt to gold can do the same in a lab. What makes bismuth special is how easy it is to get those conditions. Its low melting point and forgiving crystallization process practically guarantees hopper crystals even in a backyard setup. Compared to aluminum which requires fine control to get identical structures bismuth is a breeze. And with its oxide layer that reflects light in rainbow colors a scientific anomaly becomes a visual spectacle.
[Source]










